Abstract
Background As the most common and potentially curable non-Hodgkin lymphoma, diffuse large B-cell lymphoma(DLBCL) occurs more frequently in the elderly population, with the median age at diagnosis of 66 years old (Issa, Haematologica, 2018). According to SEER database, 5-year survival rate of DLBCL patients (pts) under 55 years old was 78%, while that of pts over 65 years old was only 54% (https://seer.cancer.gov/statfacts/html/dlbcl.html). Moreover, in western countries, 23% of elderly pts with newly diagnosed DLBCL received no immunochemotherapy, and the proportion was even higher among those aged >80 years (Hamlin, Oncologist, 2014). Well-tolerated and effective treatment for elderly/frail pts with DLBCL remains an unmet need. Thus, we conducted a clinical trial to add rituximab, lenalidomide, and zanubrutinib (ZR2) regimen to first-line therapy as a "Gentle Initiation", for purpose of reducing toxicity and chemotherapy exposure in elderly/frail pts (NCT05290090).
Methods Eligible pts had treatment-naive histologically confirmed diagnosis of DLBCL. Additional eligibility criteria included age 70 years or older (or 65-70 years old with ECOG ≥2), at least one measurable lesion, acceptable haptic and hematological function. Pts with primary CNS lymphoma, primary mediastinal large B-cell lymphoma, secondary CNS involvement were excluded. Additional exclusion criteria included history of pulmonary embolism, severe hemorrhagic disease, active infection. Once deemed eligible, pts will receive two cycles of ZR2 (21 days per cycle) and subsequently four cycles of RCHOP immunochemotherapy. Responses were assessed by CT (after ZR2) and PET-CT (end of treatment, EOT) according to the Lugano Classification 2014. Venous thromboembolism and pneumocystis pneumonia prophylaxis were required for all pts. Prophylactic G-CSF was not routinely administered during ZR2 period. The primary objective was to assess the complete response rate(CRR) at EOT.
Results Between Jan 1, 2021, and Jun 1, 2022, 26 pts were enrolled. Pts’ baseline demographics and clinical characteristics are shown in Table 1. The median age was 76 years old with a female predominance. Up to 48% of pts were assessed as frail at diagnosis. The vast majority of pts were advanced stage by Ann Arbor staging and intermediate- to high-risk by IPI. More than half pts had double-expressor lymphoma which indicated poor prognosis.
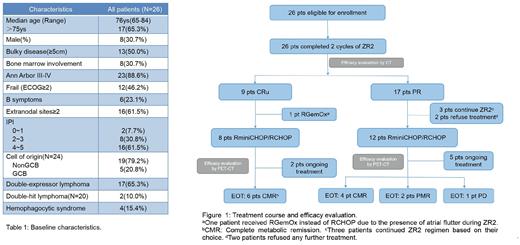
Treatment course and efficacy evaluation of all pts are summarised in Figure 1. The overall response rate (ORR) evaluated after ZR2 was 100% (CRu 34.6%, PR 65.4%). The median tumor volume reduction from baseline to completion of 2 cycles of ZR2 was 84% (55%-95%), and the responses did not differ between NonGCB and GCB DLBCL. By the time of analysis, a total of 13 pts have finished the preconcerted treatment, CRR at EOT reached 76.9% (ORR 92.3%). Besides, three pts who insisted on continuing ZR2 instead of RCHOP achieved remission at EOT (CMR 1, PMR 2).
The adverse events (AEs) during ZR2 period were analyzed. Generally, the majority of AEs were grade 1-2 (74.1%). Neutropenia and anemia were both the most common AEs (38.5%), followed by thrombocytopenia (34.6%). Moreover, neutropenia was also the most frequently observed grade 3-4 AE (23.1%). Two cases of AEs of grade 4 (neutropenia and thrombocytopenia) were relevant to preexisting hemophagocytic syndrome. As the most concerned non-hematological events, pneumonia and atrial flutter of grade 3 were observed in 2 and 1 pts, respectively. Dose reductions or dose delays of ZR2 because of serious AEs occured in 5 pts (19.2%) , but no one discontinued treatment.
With a median follow-up time of 9.8m (2.0-18.1m), the median PFS and OS were not reached. 1 year OS and PFS was 92.3% and 88.5%, respectively. Overall, 3 pts experienced disease progression (1 at EOT, 2 during follow up), 2 pts died (1 due to progressive disease, 1 due to fatal infection after the last cycle of RCHOP).
Conclusions "Gentle Initiation” with ZR2 induced satisfactory response and mild toxicities in this elderly/frail DLBCL population with multiple clinical and molecular adverse prognostic factors. Reduced cycles of RCHOP still yielded amazingly high CRR. We believe this therapeutic strategy can be regarded as a successful attempt at improving treatment for elderly pts with DLBCL, but further follow-up is still warranted for durability of responses.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Lenalidomide and zanubrutinib has not been approved for treatment of DLBCL in the first-line setting.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal